From microlitre droplets to molecular action movies
In this article, I’ll describe how scientists transform biological complexes—proteins, protein-molecule pairs, etc.—from their native environment to a controlled, low-temperature setting. By employing cutting-edge cryo-EM techniques, they manage to capture intricate structural details, including a time component.
Introduction
There are a number of shared elements in the process of generating a protein rich solution to be imaged by X-ray crystallography and CryoEM. But once we have this valuable droplet, how do we prepare it for imaging?
A three-microlitre droplet, less than 2 mm in diameter, is spread onto a grid and then blotted with filter paper to reduce the solution to a thin film of 35 nm thickness. In the previous article, we discussed the apoferritin protein used to calibrate an electron microscope. Apoferritin has a diameter of 2.5 nm, so about a dozen apoferritins stacked on top of each other would equal the thickness of the aqueous film.
The original solution is manufactured at 8 mg/mL density. If proteins were distributed uniformly in the film following the blotting, the expected number of observed proteins would have been more than ten times lower than actually observed. A spherical droplet is converted into a thinly laid-out film, dramatically increasing the air-water interface to which proteins preferentially stick. A strict mechanistic explanation is an area of ongoing investigation, but this phenomenon works in experimenters’ favour [1].
Amorphous Ice
We then proceed to flash-freeze it in liquid ethane at -180°C. It cools in a few milliseconds. This rapid cooling process creates an ice enclosure for target proteins where water molecules do not have time to orient themselves into a well-known hexagonal structure.
Why does this matter? As discussed in the previous article, electrons scattering on a spatially periodic lattice are going to generate a powerful coherent signal. Since our interest lies in imaging the atomic distances between protein atoms, not water atoms, it’s beneficial for the electron scattering against frozen water to occur in random directions, forming a uniform “background radiation.” This amorphous—vitreous—ice is technically glass, which is fitting for our microscopy endeavour.
Amorphous Carbon
Just as structured, periodic lattices for ice enclosing the proteins reduce the signal-to-noise ratio, the same is true of the material on which the frozen solution is deposited: a metal grid covered in carbon. But carbon is… graphite, carbon is diamond, carbon is graphene, carbon is fullerene—very orderly, periodic structures. How does one create carbon that’s:
Amorphous
Disorganised
Three to five nanometres thick
High purity
Adhering to the metal mesh
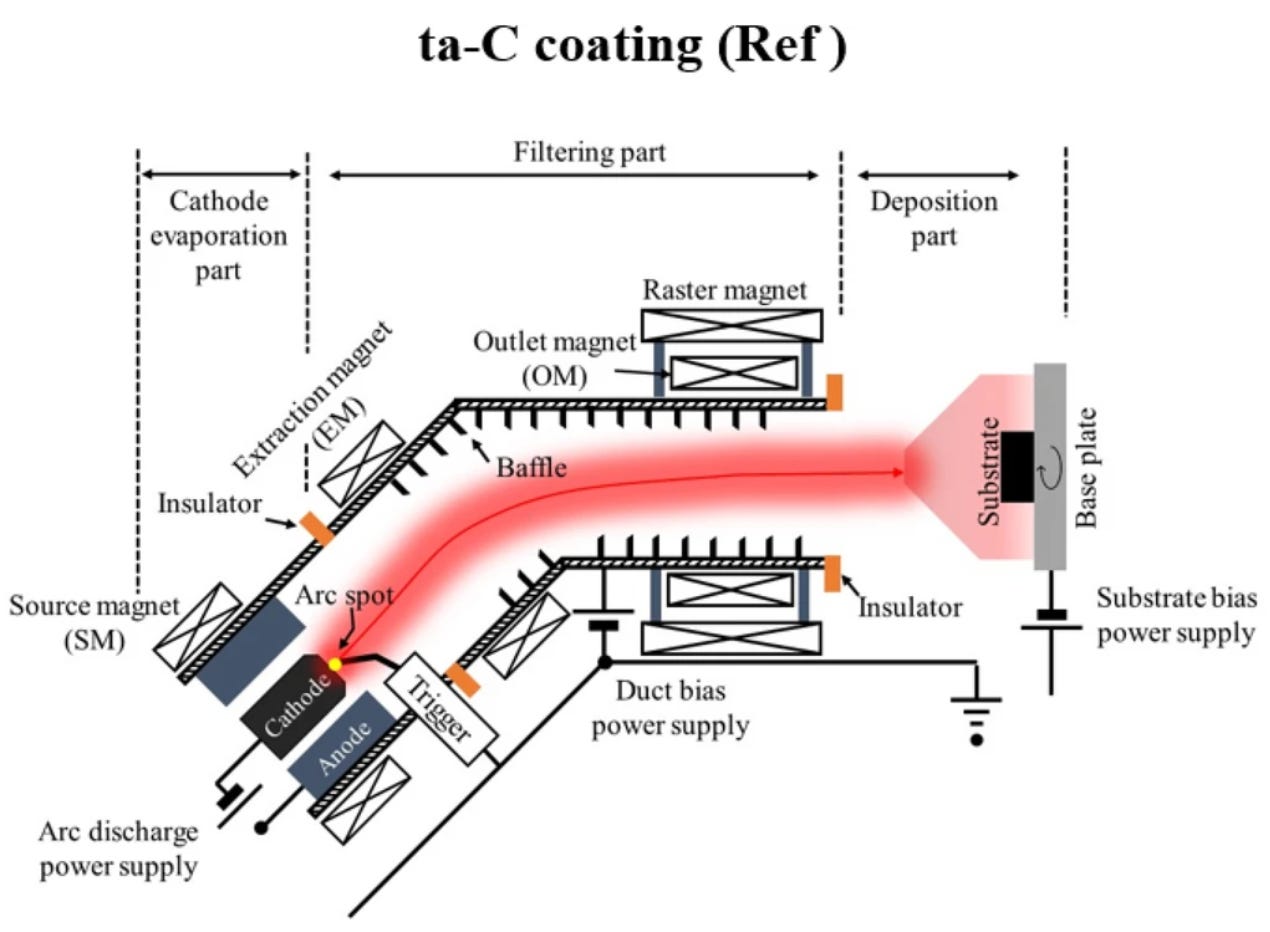
Let’s examine modern manufacturing techniques involving hot plasma and evaporation-based carbon deposition [2]. This publication describes a multitude of possible techniques; however, it concludes that Filtered Cathodic Vacuum Arc (FCVA) is the most efficient method to synthesise coherent thin films. In this process, a carbon target is treated like a cathode. Between it and the anode—separated by a few centimetres—a voltage of around 100V is applied to create an ionised carbon+ plasma. By adding the filtered component, which removes the occasionally ejected pieces of cathode material, deposition onto the substrate can be of high purity and quality.
Caged neurotransmitters
…is an excellent punk band name. They are also key to observing the dynamics of the protein-molecule system. Neurotransmitters—dopamine, serotonin, glutamate, GABA, and ATP—are manufactured in a form that’s bound to another chemical group, which, when hit with a specific wavelength of UV—there’s that quantum physics again—separates and allows the small neurotransmitter to bind to its receptor, i.e., a protein binding pocket.
What does this cage look like? A common compound is a nitrobenzyl group, which efficiently absorbs UV. The molecule arrangement looks like this:
NT - CH2 - Ph - NO2
Where NT is the neurotransmitter and Ph is the hexagon-shaped phenyl group C6H5 (benzene ring minus a hydrogen). Initially, the UV light hits the Ph-NO2 bond; however, this introduces a series of quick steps involving spatial rearrangements and further bond severance, leaving NT in its free form.
From still images to movies
It’s time to introduce caged neurotransmitters into the high concentration of proteins in a thin film solution, adhering to amorphous carbon. Scientists have automated the process of hovering the sample over the liquid ethane, bombarding it with UV to release neurotransmitters from their cages, and then flash-plunging it in a programmable time window as short as 10 ms. By repeating the experiment at different time scales, they can reconstruct the behaviour of a target protein interacting with small molecules.
In one paper [4], they release individual protons (hydrogen ions) to vary the pH of the solution from 8.0 down to 6.0, which alters the resting conformation of a cASIC1a protein’s pocket. By altering the shape, one is able to reconstruct the temporal dynamics of a protein pocket altering its shape with CryoEM snapshots.
Summary
Over the course of three articles we’ve gone through: biological applications of modern CryoEM technology. Physical and modelling complexities of electron microscopy and today we dove into the engineering of freezing and temporally evolving biological samples. It’s difficult to overstate how sophisticated the modern applied sciences are. And impossible not to feel inspired about where will they take us next.
Let me know what you found surprising, informative or muddled. Feedback is a gift.
#atoms
References
[1] Better, Faster, Cheaper: Recent Advances in Cryo-Electron Microscopy. Chua EYD, Mendez JH, Rapp M, et al. - Annu Rev Biochem. 2022
[2] A review of plasma‑assisted deposition methods for amorphous carbon thin and ultrathin films with a focus on the cathodic vacuum arc technique. Anurag Roy, Shengxi Wang, Kyriakos Komvopoulos - Journal of Materials Research 2023
[3] Thermal stability of Si/SiC/ta-C composite coatings and improvement of tribological properties through high-temperature annealing. Jang, YJ., Kim, JI., Kim, Ws. et al. - Sci Rep 2022
[4] Light-coupled cryo-plunger for time-resolved cryo-EM - Yoder N, Jalali-Yazdi F, Noreng S, Houser A, Baconguis I, Gouaux E - J Struct Biol. 2020





